Dhl air mail. FDA has issued a final guidance entitled Waiver of In-vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Citrix app for mac.

Bcs Fda

See Full List On Lubrizolcdmo.com
- Concept paper for comment WHO Drug Information Vol. 1, 2016 4 Pre-publication draft - page numbers are not for citation purposes affect the feasibility of the manufacturing process, including packaging and quality control.
- Dissolution Methods Database Search. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System.
Bcs Classification Database Management System
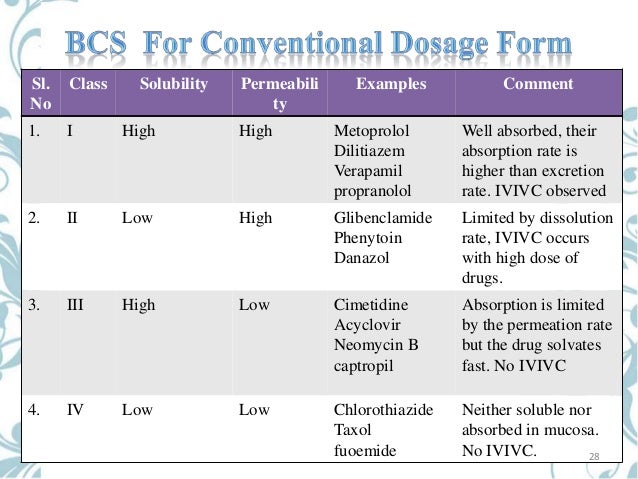
Bcs Fda
See Full List On Lubrizolcdmo.com
- Concept paper for comment WHO Drug Information Vol. 1, 2016 4 Pre-publication draft - page numbers are not for citation purposes affect the feasibility of the manufacturing process, including packaging and quality control.
- Dissolution Methods Database Search. Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System.
Bcs Classification Database Management System
GUIDANCE ON BIOPHARMACEUTICS CLASSIFICATION SYSTEM (BCS ..
| Trade Name | Source | Permeability |
|---|

